
|
MPISF - Göttingen
Abt. Molekulare
Wechselwirkungen, Projekt 407
Earlier Work on Metal Surfaces in the
Department |
 |
Helium Atom Scattering from
Surfaces:
Project 403 HUGO II
 The main aim of this project is the
investigation of the dynamics and interactions of adsorbates on single crystal
metal surfaces with helium atom scattering (HAS). In particular, the low
frequency dynamics in the thermal energy range (<25 meV, 200 cm-1)
are studied, which are important for the thermodynamics and energy accomodation
of the system under normal temperature conditions.
The main aim of this project is the
investigation of the dynamics and interactions of adsorbates on single crystal
metal surfaces with helium atom scattering (HAS). In particular, the low
frequency dynamics in the thermal energy range (<25 meV, 200 cm-1)
are studied, which are important for the thermodynamics and energy accomodation
of the system under normal temperature conditions.
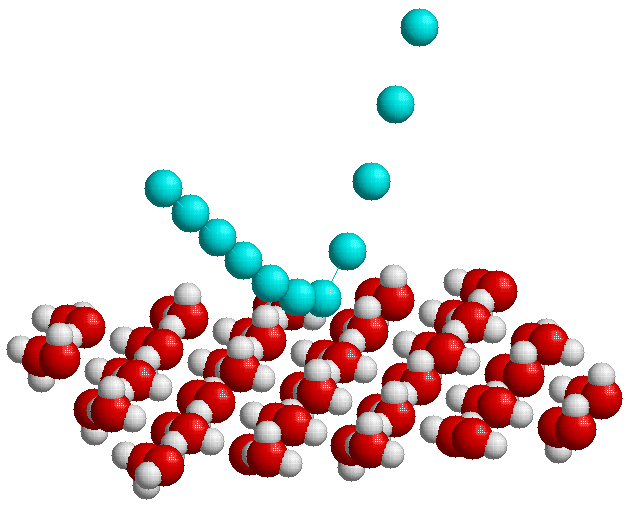
Figure 1: Schematic diagram illustrating the scattering
of helium atoms (blue) from a proton disordered ice bilayer which forms the top
layer of a thin ice film grown on Pt(111). The helium atoms do not penetrate the
surface and are scattered approximately 3Å from the surface.
Recent experiments have focussed on determining:
- The surface
phonons of thin molecular films, e.g., ice and noble gas films grown on
Pt(111).
- The monolayer
film modes, e.g., CO on Cu(001), noble gases and organic molecules.
- The low
frequency translational vibrations of isolated atoms and molecules, e.g.,
NO, CO and H2O on Pt(111).
- The diffusion
of atoms and molecules on flat metal surfaces, e.g., CO, H, Na and Xe on
Pt(111).
From these measurements it has been possible to construct potential
energy surfaces which completely describe the motion of the atom or molecule
on the surface and the interactions between them.
In a high resolution
helium suface scattering apparatus, such as HUGO II, a well collimated beam of
nearly mono-energetic helium atoms is scattered from the sample surface. The
mono-energetic helium atom beam is produced by supersonic expansion of helium
atoms from a high pressure (~200bar) source through a small orafice (~0.01mm
diameter) into a vacuum. A helium sensitive detector located about 1.4 meters
from the sample detects the helium atoms scattered at a fixed total scattering
angle, i.e., incident + final angles. In order to measure the dynamics of the
adsorbate covered surface the incident helium atom beam is chopped into short
time pulses and the flight time to the detector is measured. From a series of
time-of-flight (TOF) measurements made over a range of incident and final
scattering angles the phonon dispersion curves and other information about the
dynamics can be obtained.
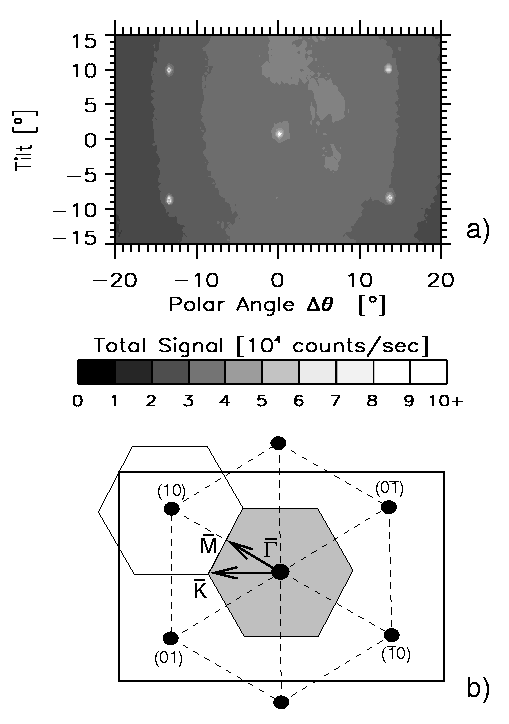
Figure 2: (a) A two-dimensional
helium diffraction pattern obtained from a 100nm thick crystalline ice layer
grown on Pt(111). The incident beam energy was 10.4 meV and the ice surface
temperature was 45K (-228°C). The positions of the diffraction peaks (white
points) describe a hexagonal reciprocal unit cell of dimension
1.605Å-1 (b) equivalent to a real-space unit cell dimension of 4.52Å.
This unit cell size and shape corresponds with that of the proton disordered ice
surface shown in Figure 1.
The incident and final
scattering angles are varied by rotating and tilting the sample. The incident
energy of the helium atoms can also be changed simply by increasing or
decreasing the temperature of the supersonic source allowing a large range of
scattering conditions to be investigated. For example, by systematically
changing the sample rotation and tilt it is possible to produce a
two-dimensional diffration pattern of the helium atoms scattered from a surface,
as shown in Figure 2.
The vibrational
characteristics of the surface layer atoms and molecules of an ordered film
differ from those of the bulk due to the reduced coordination (fewer neighbors)
at the surface. This can lead to a reconstruction, or reordering, of the
surface, relaxation of the atoms from their ideal bulk positions and enhanced
vibrational amplitudes coupled with weaker bonding.
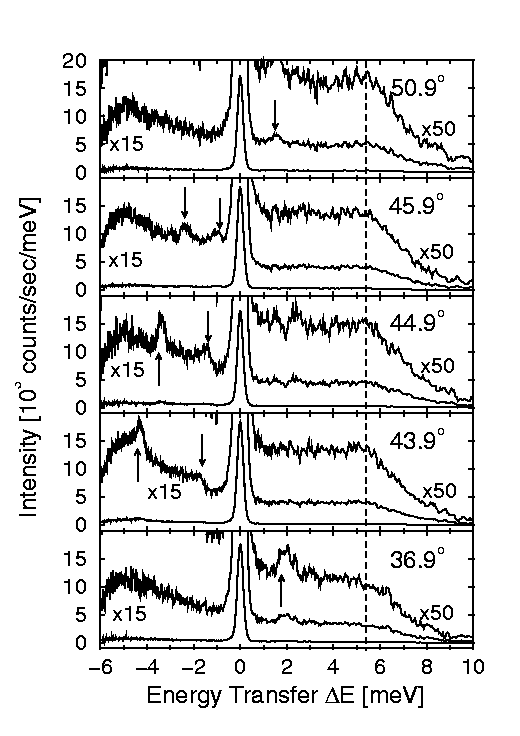
Figure 3: Several helium time-of-flight (TOF) spectra from the ice
surface for different scattering angles. The incident helium atom energy was
10.4 meV and the surface temperature was 45K (-228°C). The spectra have been
transformed from flight time into energy transferred from the helium atom to the
ice surface. The peak at zero energy transfer corresponds to diffuse elastic
scattering from surface defects. Several other peaks, which are marked by
arrows, can be seen which change position with incident angle (shown in the top
right hand corner of each spectrum).
Helium atom scattering
provides two useful pieces of information about the difference between the
surface and the bulk of a crystal. Firstly, because the wave length of the
helium atoms is comparable with the distance between atoms or molecules on the
surface, atom diffraction can be used to determine the degree of reconstruction
of the surface relative to the bulk by providing the size and shape of the
surface unit cell, the smallest repeatable configuration of the atoms or
molecules at the surface. Figure 2 shows such a diffraction pattern of the ice
surface as grown on a Pt(111) surface, highlighting the hexagonal shape of the
surface unit cell and providing the distance between protruding water molecules.
The second useful piece of information derives from the vibrations of
the surface molecules, which are observed in time-of-flight (TOF) experiments,
as described earlier. Figure 3 shows a typical set of TOF spectra for scattering
from the ice surface at low temperature. The spectra have been converted from
flight time to energy transfered from the helium atom to the surface during the
scattering process, i.e., negative energy transfer corresponds to a slower final
helium atom velocity as compared with its initial velocity. From the peaks in
the spectra the surface phonon dispersion curves can be derived and compared
with calculations of the surface dynamics using known interaction potentials.
The surface phonon dispersion curves of ice are shown in Figure 4.
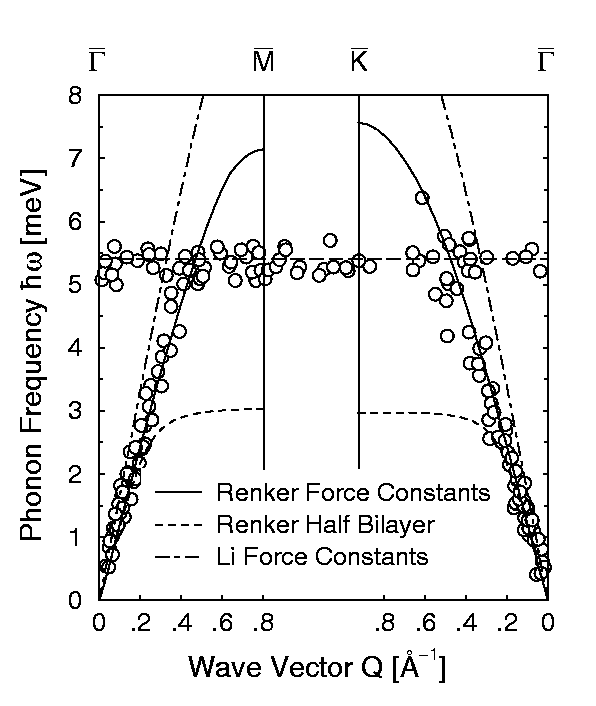
Figure 4: The phonon dispersion curves of the ice surface
derived from helium atom scattering time-of-flight measurements (open circles).
The solid lines show the results of calcualtions of the dynamics using
interaction potentials dervided from bulk measurements using inelastic neutron
scattering.
Similar measurements have also been undertaken
for the surfaces of 100nm thick xenon and krypton films grown on Pt(111). Helium
atom scattering is particularly useful for measurements of this type because the
helium atoms are neutral and the impact energies are very low, comparable with
the single phonon energies, so that the surface does not become charged or
disturbed.
For further information on the structure and
phonons of the ice surface:
- Structure and Phonons of the Ice Surface, J. Braun et
al., Phys. Rev. Lett. 80, 2638 (1998).
- Orientational Ordering of Two-Dimensional Ice on Pt(111) A.
Glebov et al., J. Chem. Phys. 106, 9382 (1997).
- A Helium Atom Scattering Study of the Structure and Dynamics of the
Ice Surface, A. Glebov et al., to be
published (gzipped postscript file 407kB).
and for general
information on helium atom scattering investigations of surface phonons see,
e.g.,
- Experimental Determination of Surface Phonons by Helium Atom
Scattering and Electron Energy Loss Spectroscopy, J.P. Toennies, Springer
Series in Surface Sciences 21 (Springer Verlag Berlin, Heidelberg
1991), 111.
The low frequency
vibrational characteristics of a monolayer adsorbate film provide a great deal
of information about the binding of the molecules to the surface and the
interactions between the molecules. For adsorbed atoms there are three phonon
modes corresponding to the three translational degrees of freedom. These are
divided into modes which are perpendicular to the monolayer plane and which are
typically affected only a small amount by the presence of the other adsorbed
atoms. There are also in-plane vibrations of the atoms parallel to the monolayer
which depend strongly on the adsorbate-adsorbate interaction strength and
registry with the surface. For adsorbate overlayers which are commensurate with
the metal substrate, i.e., the adsorbed atoms are held in well defined sites on
the metal surface, then the forces holding the molecule in that site also
contribute to the parallel motion. This is typically observed as a finite
vibrational frequency when all atoms are moving in-phase parallel to the surface
(wave vector Q=0) and the inter-atomic distance does not change. For
incommensurate adsorbate layers, which are not in registry with the surface, the
substrate plays no role and the frequency for in-phase motion is zero.
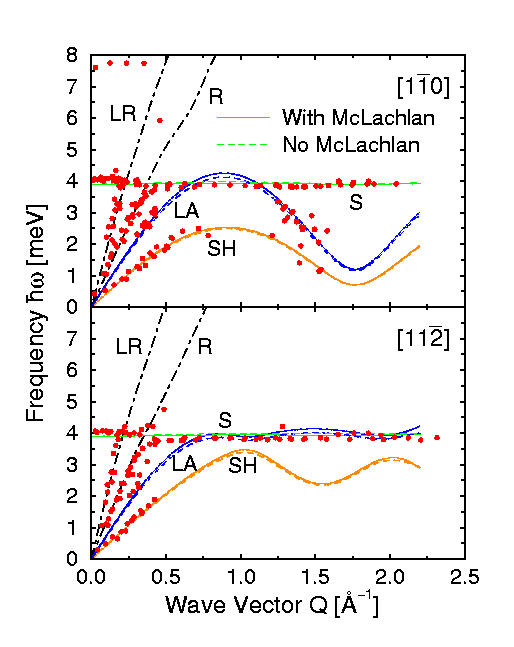
For
example, Figure 5 shows the phonon dispersion curves of an incommensurate
monolayer of krypton adsorbed on Pt(111) obtained from an extensive series of
time-of-flight measurements. The experimental data points are shown by the red
dots. In addition to the low frequency parts of the Pt(111) surface Rayleigh and
longitudinal phonon branches (black dot-dashed lines) three modes of
the krypton layer can be observed which can be accurately modeled using
gas-phase Kr-Kr potentials. They are; (a) the vibration of the krypton atoms
perpendicular to the surface in the Kr-Pt potential (green line), (b) the
in-plane longitudinal acoustic (LA) mode which is a compression wave within the
krypton film (blue line), and (c) the in-plane shear-horizontal (SH) mode which
is a transverse shearing motion of the krypton atoms within the monolayer
(orange line).
Figure 5: The phonon dispersion curves of a monolayer
of krypton on a Pt(111) surface measured with helium atom scattering. The
surface temperature was 50K (-223°C) and the incident helium beam energy was 8
meV. The solid lines show the results of calculations of the dynamics using
unmodified gas-phase krypton-krypton potentials. The orange lines show the shear
horizontal modes (SH), the blue lines the longitudinal acoustic modes (LA) and
the green lines the perpendicular vibrations, or atom-surface bond stretch mode
(S).
References:
- A Helium Atom Scattering Study of the Growth and Dynamics of
CH4 and C2H6 on Cu(001), A.P. Graham
et al., J. Chem. Phys. 106, 2502 (1997).
- Adsorption, desorption, monolayer structure and dynamics of
C2H4 on Cu(001), A.P. Graham et al., J.
Chem. Soc., Faraday Trans. 92, 4749 (1996).
- Vibrations of the Commensurate Monolayer Solid Xe/Pt(111), L.W.
Bruch, A.P. Graham and J.P. Toennies, Molecular Physics 95, 579 (1998).
- The Low-Frequency Vibrational Modes of c(4x2) CO on Pt(111), A.P.
Graham, J. Chem. Phys. 109, 9583 (1998).
- The Dispersion Curves of the Three Phonon Modes of Xenon, Krypton and
Argon Monolayers on the Pt(111) Surface, L.W. Bruch, A.P. Graham and J.P.
Toennies, submitted to J. Chem. Phys.
For an
isolated adsorbed atom or molecule, the external vibrational dynamics are
completely determined by the bonding to the surface. Thus, a comparison of the
vibrational properties of isolated adsorbates with those of the denser
monolayers can provide additional information concerning the effect of
inter-molecular interactions. In addition, if the molecule-surface bond is
complicated by effects such as charge redistribution or transfer to or from the
surface, then the monolayer dynamics become more difficult to simulate. Further,
for isolated atoms or molecules, calculations of the bonding and dynamics can be
made on small metal clusters which simulate the metal surface and electronic
structure. An example for carbon monoxide adsorption on Cu(001) is; P.S. Bagus and Ch. Wöll, Chemical Physics Letters 294, 599
(1998).
 Helium atom
scattering is particularly useful for studying the very low frequency parallel
vibration frequencies of adsorbates such as CO and NO because of its high
resolution, typically 0.3 meV (3cm-1), and large cross-section for
scattering from adsorbates, which gives it a high sensitivity to low adsorbate
densities. Due to the parallel motion of these modes they are difficult to
detect with electron energy loss spectroscopy (EELS) and their frequency is too
low to measure with infrared spectroscopy (IRAS).
Helium atom
scattering is particularly useful for studying the very low frequency parallel
vibration frequencies of adsorbates such as CO and NO because of its high
resolution, typically 0.3 meV (3cm-1), and large cross-section for
scattering from adsorbates, which gives it a high sensitivity to low adsorbate
densities. Due to the parallel motion of these modes they are difficult to
detect with electron energy loss spectroscopy (EELS) and their frequency is too
low to measure with infrared spectroscopy (IRAS).
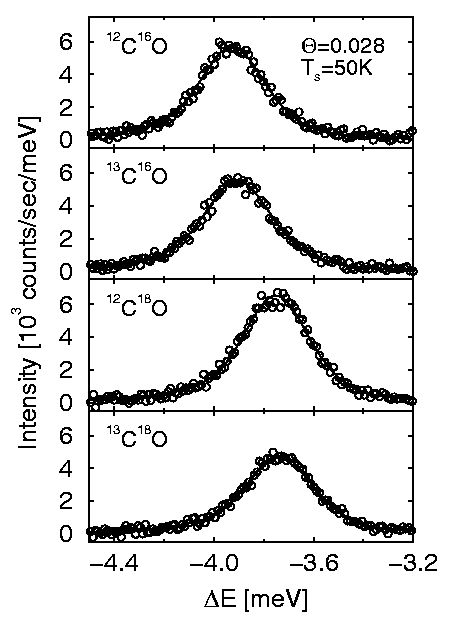
Aside from the
vibrational frequency itself, helium scattering time-of-flight measurements can
also be used to determine the details of the vibrational motion of the molecule
via isotope substitution. Figure 6 shows sections of time-of-flight spectra,
corresponding to the parallel mode peak of 3% CO on Cu(001) for the four
different CO isotopomers 12C16O,
13C16O, 12C18O and
13C18O. The frequency shifts strongly when the oxygen
isotope is changed but only slightly when the carbon isotope is replaced. This
indicates that the oxygen atoms are vibrating with larger amplitudes than the
carbon atoms so that the molecule is not purely translating, but also rotating
as it moves.
Figure 6: Helium atom scattering time-of-flight spectra
for about 3% CO on Cu(001) at a surface temperature of 50K. The spectra have
been transformed from flight time to energy loss and the figure shows the energy
loss range corresponding to the parallel vibrational frequency of the CO
molecules. The frequency is much lower for the heavier oxygen isotopes compared
with the carbon isotopes.
In addition to providing
information on the precise motion of the adsorbate the time-of-flight
measurements also supply the rate of damping of the energy of the vibrational
mode to the substrate. The damping gives the vibration a finite lifetime which
leads to a broadening of the vibrational peaks. The peak broadening, and hence
the lifetime, can be accurately extracted taking account of the finite
instrument resolution. In this way the lifetime of the parallel vibration of 3%
CO/Cu(001) was determined to be 8ps. A comparison with theoretical simulations
indicates that the damping is almost entirely due to the creation of substrate
vibrations rather than excitation of the electrons at the surface.
References:
- High-Resolution Helium Atom Time-of-Flight Spectroscopy of
Low-Frequency Vibrations of Adsorbates, F. Hofmann and J.P. Toennies,
Chemical Reviews 96, 1307 (1996).
- Observation of the Broadening And Shift of the Frustrated Translation
Vibrational Mode of CO on Cu(001) by High Resolution Helium Atom Scattering
, A. Graham, F. Hofmann and J.P. Toennies, J. Chem. Phys. 104,
5311 (1996).
- Experimental Determinaton of the Vibrations and Diffusion of Isolated
CO Molecules Moving Parallel to a Pt(111) Surface, A.P. Graham and J.P.
Toennies, Europhys. Lett. 42, 449 (1998).
- Phonons on Surfaces: The Importance of Structure and Adsorbates,
Ch. Wöll, Appl. Phys. A53, 377 (1991).
- A He-Atom Scattering Study of the Frustrated Translational Mode of CO
Chemisorbed on Defects on Copper Surfaces, J. Braun et al., J.
Chem. Phys. 105, 3258 (1996).
Many
important physical processes depend on diffusion of adsorbates on surfaces. For
example, the diffusion of carbon monoxide on the surfaces of the particles in an
automobile catalytic converter allows them to find adsorbed oxygen atoms and
form the non-toxic carbon dioxide. The diffusion of metal atoms on surfaces
permits the growth of well ordered metal layers which are used, for example, to
make electrical contacts on semiconductor devices.
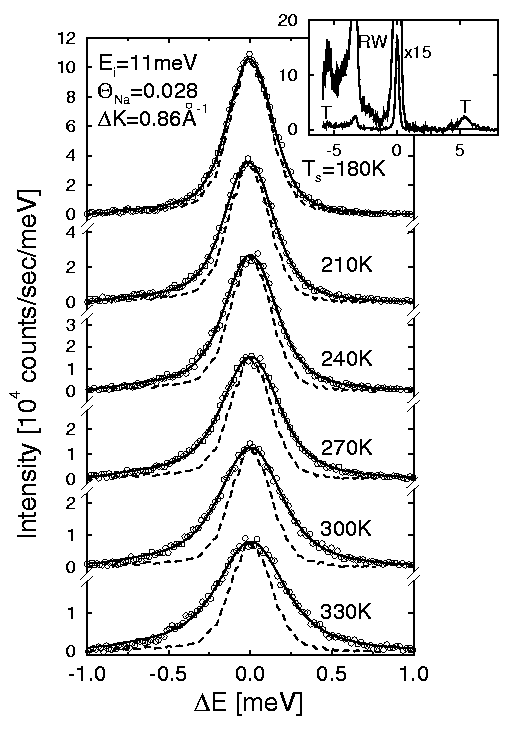 Figure 7: A series of spectra demonstrating the energetic broadening of the
elastically scattered helium atoms due to diffusional motion of 3% sodium atoms
on a Cu(001) surface. The broadening of the experimental peak (open circles)
becomes much larger than the intrinsic instrument width (dashed line) as the
surface temperature is increased due to the increased activity of the sodium
atoms on the surface. These and other measurements show that the sodium atoms
diffuse by jumping from one site to another on the well ordered copper surface
and provide information on the energy required to make the jump, i.e., the
activation energy.
Figure 7: A series of spectra demonstrating the energetic broadening of the
elastically scattered helium atoms due to diffusional motion of 3% sodium atoms
on a Cu(001) surface. The broadening of the experimental peak (open circles)
becomes much larger than the intrinsic instrument width (dashed line) as the
surface temperature is increased due to the increased activity of the sodium
atoms on the surface. These and other measurements show that the sodium atoms
diffuse by jumping from one site to another on the well ordered copper surface
and provide information on the energy required to make the jump, i.e., the
activation energy.
Helium atom scattering has proved to be a
versatile tool for the investigation of surface diffusion processes. Due to its
high sensitivity for surface defects, the diffuson of very small quantities of
adsorbates on a surface can be investigated. Further, helium scattering has been
used successfully to probe the motions of a variety of very different
adsorbates, e.g., hydrogen, xenon, carbon monoxide, and sodium as well as the
self diffusion, or surface premelting, of a number of different surfaces
including semi-conductors.
The diffusional motion of the adsorbates on a
surface induces an energy broadening of the elastically scattered helium atoms.
The movement of the adsorbate induces a phase shift in the helium atoms
scattering from it which corresponds to the transfer of a small amount of energy
akin to the Doppler frequency shift heard from a moving vehicle. For diffusion
rates more than about 10-6cm2/sec the energetic broadening
can be distinguished from the intrinsic elastic instrument peak width and
monitored as a function of surface temperature and scattering angle. Figure 7
shows a series of time-of-flight spectra of the quasi-elastic peak for
helium atoms scattering from a small coverage of sodium atoms on a Cu(001)
surface. The peak becomes progressively broader as the surface temperature is
raised from 180K to 330K due to the diffusion of the sodium atoms on the copper
surface.
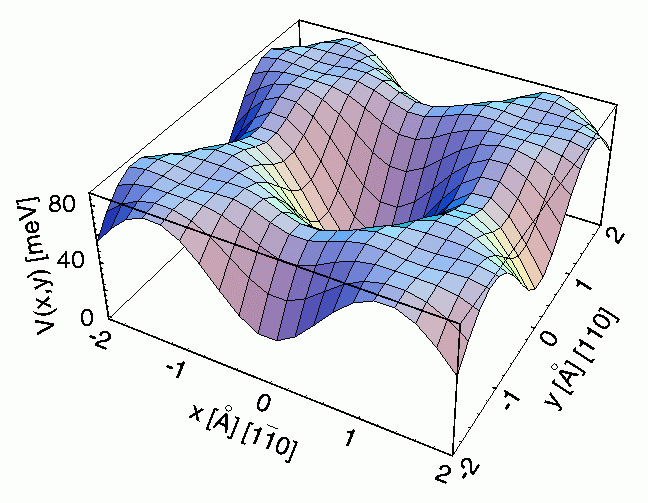 Figure 8: The potential surface determining the lateral motions of sodium
atoms on a Cu(001) surface. The potential was obtained by comparing the
simulated motions of sodium atoms on the surface to the results of quasi-elastic
helium atom scattering experiments.
Figure 8: The potential surface determining the lateral motions of sodium
atoms on a Cu(001) surface. The potential was obtained by comparing the
simulated motions of sodium atoms on the surface to the results of quasi-elastic
helium atom scattering experiments.
The unique combination of
diffusion and vibrational information provided by helium scattering
time-of-flight measurements permits the lateral potential energy surface of an
adsorbate on a substrate to be accurately described. The potential energy surface is an
effective landscape on which the atoms and molecules move, with valleys
representing the preferred adsorption locations and the hills determining the
movement on adsorbates from one valley to the next. The curvature of the valley
determines the vibrational frequency of the atom or molecule in its preferred
site similar to the motion of a ball in a round bowl. In Figure 8 the lateral
potential surface for sodium atoms on a copper surface is shown. The middle is
indented and corresponds to the optimum sodium atom adsorption place on Cu(001).
The sodium atoms can leave that site in any direction if they have enough
energy. The height of the potential for the different directions was determined
by rotating the copper surface so that the helium atoms `look' along that
direction and comparing the results with simulations of the motions of the
sodium atoms.
References:
- Determination of the Na/Cu(001) Potential Energy Surface from Helium
Scattering Studies of the Surface Dynamics, A.P. Graham et al.,
Phys. Rev. Lett. 78, 3900 (1997).
- Experimental and Theoretical Investigation of the Microscopic
Vibrational and Diffusional Dynamics of Sodium Atoms on a Cu(001)
Surface, A.P. Graham et al., Phys. Rev. B56, 10567
(1997).
- Elementary Processes of Surface Diffusion Studied by Quasielastic
Helium Atom Scattering, A.P. Graham, W. Silvestri and J.P. Toennies in
Surface Diffusion: Atomistic and Collective Processes, M.C. Tringides
ed. (Plenum Press, New York, 1997) p565.
- Determination of the Lateral Potential Energy Surface of Single
Adsorbed Atoms and Molecules on Single Crystal Surfaces using Helium Atom
Scattering, A.P. Graham and J.P. Toennies, Surf. Sci. 427-8, 1
(1999).
- Behavior of Single Adatoms on the Ge(111) Surface above the 1050K
Phase Transition82, 3300 (1999).
- Quasielastic Helium Atoms Scattering from a Two-dimensional Gas of Xe
Atoms on Pt(111), J. Ellis, A.P. Graham and J.P. Toennies, Phys. Rev.
Lett. 82, 5072 (1999).
- Quasielastic Helium Atom Scattering Measurements of Microscopic
Diffusional Dynamics of H and D on the Pt(111) Surface, A.P. Graham, A.
Menzel and J.P. Toennies, J. Chem. Phys. 111, 1676 (1999).
- Quasielastic Helium Atom Scattering Measurements of Microscopic
Diffusion of CO on the Ni(110) Surface, M.F. Bertino et al., J.
Chem. Phys. 105, 11297 (1996).
- Quasielastic Helium Scattering Studies of Adatom Diffusion on
Surfaces, J.W.M. Frenken and B.J. Hinch in Helium Scattering from
Surfaces, Springer Series in Surface Sciences 27, E. Hulpke ed.
(Springer-Verlag Berlin, Heidelberg, 1992) p287.
- Self-Diffusion at a Melting Surface Observed by He Scattering,
J.W.M. Frenken, J.P. Toennies and Ch. Wöll, Phys. Rev. Lett. 60, 1727
(1988).
Former Project Members:
- Dr. Walter Silvestri, M.P.I. für Festkorperforschung, Stuttgart, Germany.
- Dr. Alexander Menzel, Dept. of Physical Chemistry, Innsbruck, Austria.
- Prof. Massimo Bertino, Dept. of Physics, Univ. of Missouri-Rolla, U.S.A.
(from 1.1.2000)
- Peter Hahn, Fraunhofer Institut, Freiburg, Germany.
- Dr. Frank Hofmann, Daimler-Crysler, Stuttgart, Germany.
International Collaborators:
- Prof. L.W. Bruch, Physics Dept., Univ. of Wisconsin-Madison, Madison WI,
U.S.A.
- Dr B. Gumhalter, Inst. of Physics, Zagreb Univ., Zagreb, Croatia.
- Prof. J.R. Manson, Physics Dept., Clemson Univ., Clemson SC, U.S.A.
- Prof. K.T. Tang, Physics Dept., Pacific Lutheran Univ., Tacoma WA, U.S.A.
- Dr. J. Ellis, Cavendish Lab., Cambridge Univ., Cambridge, U.K.
- Dr. D. Fuhrmann, Univ. of Missouri-Columbia, Columbia MO, U.S.A.
Back to Home Directory
© Max-Planck-Institut für Strömungsforschung, Göttingen, Germany.
Page contents and design: A.P. Graham (Updated October 7 1999)




 The main aim of this project is the
investigation of the dynamics and interactions of adsorbates on single crystal
metal surfaces with helium atom scattering (HAS). In particular, the low
frequency dynamics in the thermal energy range (<25 meV, 200 cm-1)
are studied, which are important for the thermodynamics and energy accomodation
of the system under normal temperature conditions.
The main aim of this project is the
investigation of the dynamics and interactions of adsorbates on single crystal
metal surfaces with helium atom scattering (HAS). In particular, the low
frequency dynamics in the thermal energy range (<25 meV, 200 cm-1)
are studied, which are important for the thermodynamics and energy accomodation
of the system under normal temperature conditions.




 Helium atom
scattering is particularly useful for studying the very low frequency parallel
vibration frequencies of adsorbates such as CO and NO because of its high
resolution, typically 0.3 meV (3cm-1), and large cross-section for
scattering from adsorbates, which gives it a high sensitivity to low adsorbate
densities. Due to the parallel motion of these modes they are difficult to
detect with electron energy loss spectroscopy (EELS) and their frequency is too
low to measure with infrared spectroscopy (IRAS).
Helium atom
scattering is particularly useful for studying the very low frequency parallel
vibration frequencies of adsorbates such as CO and NO because of its high
resolution, typically 0.3 meV (3cm-1), and large cross-section for
scattering from adsorbates, which gives it a high sensitivity to low adsorbate
densities. Due to the parallel motion of these modes they are difficult to
detect with electron energy loss spectroscopy (EELS) and their frequency is too
low to measure with infrared spectroscopy (IRAS). Figure 7: A series of spectra demonstrating the energetic broadening of the
elastically scattered helium atoms due to diffusional motion of 3% sodium atoms
on a Cu(001) surface. The broadening of the experimental peak (open circles)
becomes much larger than the intrinsic instrument width (dashed line) as the
surface temperature is increased due to the increased activity of the sodium
atoms on the surface. These and other measurements show that the sodium atoms
diffuse by jumping from one site to another on the well ordered copper surface
and provide information on the energy required to make the jump, i.e., the
activation energy.
Figure 7: A series of spectra demonstrating the energetic broadening of the
elastically scattered helium atoms due to diffusional motion of 3% sodium atoms
on a Cu(001) surface. The broadening of the experimental peak (open circles)
becomes much larger than the intrinsic instrument width (dashed line) as the
surface temperature is increased due to the increased activity of the sodium
atoms on the surface. These and other measurements show that the sodium atoms
diffuse by jumping from one site to another on the well ordered copper surface
and provide information on the energy required to make the jump, i.e., the
activation energy. Figure 8: The potential surface determining the lateral motions of sodium
atoms on a Cu(001) surface. The potential was obtained by comparing the
simulated motions of sodium atoms on the surface to the results of quasi-elastic
helium atom scattering experiments.
Figure 8: The potential surface determining the lateral motions of sodium
atoms on a Cu(001) surface. The potential was obtained by comparing the
simulated motions of sodium atoms on the surface to the results of quasi-elastic
helium atom scattering experiments.